42 fda guidance use of symbols on labels
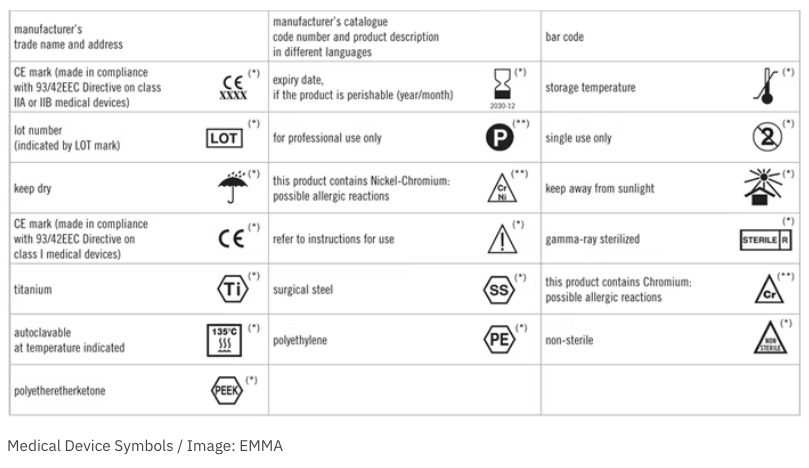
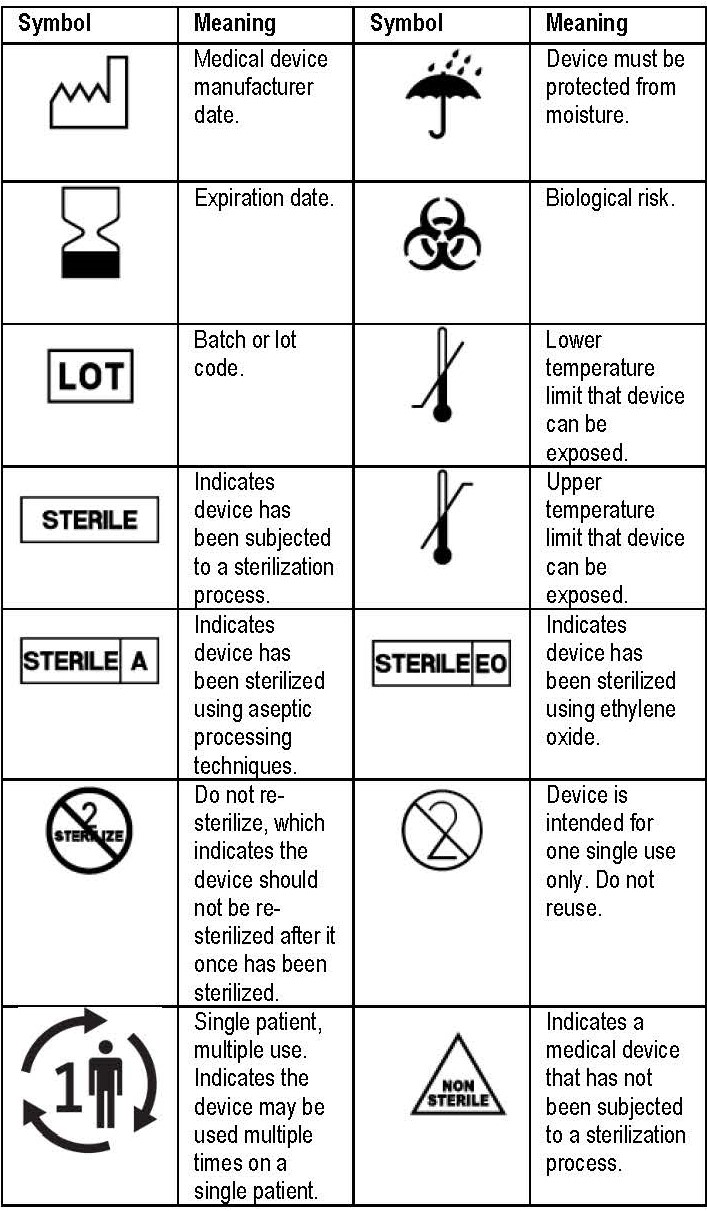
Draft Guidance for Industry and FDA Staff; Use of Symbols on Labels and ... This document provides guidance on the use of selected symbols in place of text to convey some of the information required for in vitro diagnostic devices (IVDs) intended for professional use by FDA's labeling requirements for IVDs. This draft guidance is not final nor is it in effect at this time. DATES: PHILIPS EPIQ 7 USER MANUAL Pdf Download | ManualsLib 5. Use the trackball to position the caliper for the first end point and click to anchor it. 6. Use the trackball to position the caliper for the second end point. The results update as the distance between the calipers changes. EPIQ 7 User Manual 4535 617 25341... Page 169: Measuring M-Mode Distance 1. Obtain the image you want to measure and ...
Labeling Symbols In Medical Devices Packing - Kmed-Leading infusion set ... The European Commission (EC), on the other hand, requires the use of "The Medical Devices Regulation" (MDR) issued in November 2021. This present guidance titled "Use of Symbols to Indicate Compliance with the MDR November 2021" has been updated to align with the new ISO 15223-1:2021.

Fda guidance use of symbols on labels
2 Overview of the Drug Development, Regulation, Distribution, and Use … The drug system encompasses four main stages—research and development; regulatory review; medication manufacturing, distribution, and marketing; and medication use—that each contain multiple critical control points at which quality, safety, and efficacy can be addressed, and at which breakdowns can occur. This chapter provides an overview of the major components of … Annals of the American Thoracic Society - ATS Journals Use footnotes only on the title page and in tables. Do not use footnotes in the text. Footnote symbols, in the order in which they should be used, are *, †, ‡, §, ||, ¶, **, ††, ‡‡, and so on. Do not use numbers or letters. Legends for Figures and Illustrations. The figure legends page should start on a new page. Each figure ... Federal Register :: Use of Symbols in Labeling accordingly, this final rule provides that a stand-alone symbol is allowed to be used in device labeling if: (1) the symbol is established in a standard developed by an sdo; and (2) the standard is recognized by fda under its authority under section 514 (c) of the fd&c act and the symbol is used according to the specifications for use of the …
Fda guidance use of symbols on labels. PDF Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Device ... Guidance for Industry and FDA Staff Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use Document issued on: November 30, 2004 The draft of this document was issued on October 28, 2003. The information collection provisions in this guidance have been approved under OMB control number 0910-0553. FDA Issues Final Rule Permitting Use of Symbols on Device Labeling Three years ago, FDA proposed a rule that would permit device manufacturers to use stand‑alone symbols on device labeling. In the meantime, this issue has continued to fester. So it is good news that FDA finalized the rule on June 15, 2016. Under the final rule, device manufacturers may use symbols on device labeling in one of the following ways: FDA Issues Final Rule on Symbols for Device Labels | RAPS In the past, FDA prohibited companies from using standalone symbols on device and IVD labels, and required symbols on the labels to be accompanied by explanatory text. However, in 2013, after pressure from the medical device industry, FDA issued a proposed rule that would allow standalone symbols to appear on device labels for public comment. PDF Use of Symbols on Medical Device Labels - FDA Requirements - Elsmar MIREGMGR 23rd June 2011 07:07 PM Re: Use of Symbols on Medical Device Labels - FDA Quote: In Reply to Parent Post by adv_webdev (Post 439482) We make medical devices (non-IVD, class 2, some 1) that sell in US as well as in EU and other
Runequest rpg pdf - simls.planetcanine.shop 15.02.2022 · RuneQuest are stamped with the symbols of the Red Goddess. Before the Lunar Conquest, silver coins called guilders were minted by the various city guilds in Sartar and Pavis. All these coins are roughly equivalent in value. A cow is worth about 20 L. The first coinage of the world was gold, brought to the people by the enigmatic Gold Wheel Dancers. In their honor, … FDA Voices | FDA - U.S. Food and Drug Administration Insights from FDA leadership and experts into the agency's work on policy, consumer safety & enforcement, medical products, food, & tobacco. Prescription drug - Wikipedia A prescription drug (also prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practicing medicine without a license and … Use of Symbols in Labeling | FDA Use of Symbols in Labeling The Food and Drug Administration (FDA) issued a final rule, Use of Symbols in Labeling, June 15, 2016, that became effective September 13, 2016. The final rule permits...
Understanding Food Labels | The Nutrition Source | Harvard T.H. The FDA has approved 12 health claims on food labels such as the relationship between calcium and osteoporosis; sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and folic acid and neural tube defects. However, just because a food contains a specific nutrient that is associated with a decreased risk of disease does not necessarily make the food … Use of Symbols in Medical Device Labeling- FDA's Final Rule The rule finalizes the FDA's 2013 proposed rule and affects biologics, IVDs, and medical devices regulated by 21 CFR Parts 660, 801, and 809. The revision allows for the inclusion of symbols in labeling without adjacent explanatory text (referred to as "stand-alone symbols"), so long as certain requirements are met. Child-Resistant Packaging Statements in Drug Product Labeling This guidance is intended to assist applicants, manufacturers, packagers, and distributors (collectively referred to as firms) who choose to include child-resistant packaging (CRP) IFU for Medical Devices, a Definitive Guide (EU & US) - INSTRKTIV Although not mentioned in the FDA Guidance document, page numbering and an index are also useful ways to help users navigate through the instructions for use (IFU). When you have a dynamic content delivery (e.g. instructions in the form of online help), a search bar, links to related topics, individualized delivery and context sensitive delivery, these are ways that support …
Recognized Consensus Standards - Food and Drug Administration Recognized Consensus Standards. This document specifies symbols used to express information supplied for a medical device. This document is applicable to symbols used in a broad spectrum of medical devices, that are available globally and need to meet different regulatory requirements. These symbols can be used on the medical device itself, on ...
FDA Issues Final Rule on Use of Stand-Alone Symbols in ... - Lexology On June 14, 2016, the Food and Drug Administration ("FDA" or "the agency") issued a final rule entitled Use of Symbols in Labeling. Historically, FDA regulations have only permitted use of symbols...
Federal Register :: Use of Symbols in Labeling accordingly, this final rule provides that a stand-alone symbol is allowed to be used in device labeling if: (1) the symbol is established in a standard developed by an sdo; and (2) the standard is recognized by fda under its authority under section 514 (c) of the fd&c act and the symbol is used according to the specifications for use of the …
Annals of the American Thoracic Society - ATS Journals Use footnotes only on the title page and in tables. Do not use footnotes in the text. Footnote symbols, in the order in which they should be used, are *, †, ‡, §, ||, ¶, **, ††, ‡‡, and so on. Do not use numbers or letters. Legends for Figures and Illustrations. The figure legends page should start on a new page. Each figure ...
2 Overview of the Drug Development, Regulation, Distribution, and Use … The drug system encompasses four main stages—research and development; regulatory review; medication manufacturing, distribution, and marketing; and medication use—that each contain multiple critical control points at which quality, safety, and efficacy can be addressed, and at which breakdowns can occur. This chapter provides an overview of the major components of …











.png.aspx)







.png.aspx)






Post a Comment for "42 fda guidance use of symbols on labels"